
Biopure on Verge of Biotech Holy Grail
Biopure on Verge of Biotech Holy Grail
By Hal Plotkin
CNBC.com Silicon Valley Correspondent
There could be some very good news for biotechnology investors just around the corner.
If analysts are right, Cambridge, Mass.-based Biopure Inc. {BPUR} is less than a year away from achieving the long-awaited goal of obtaining government permission to start selling the first practical human blood substitute.
According to a variety of sources, the company is nearing the apparently successful completion of the final Phase III stage of testing its new product, called Hemopure, and has begun to prepare the data needed to file a Biologic Licensing Application with the Food and Drug Administration.
Analysts warn that its still possible Biopure’s new product might yet prove a disappointment.
But they add that all signals indicate that is now unlikely, given the very late stage of Biopure’s product-development process. As such, several analysts say they expect the company’s stock to move up rather sharply over the near term, with some saying the stock could more than double over the next year, assuming the new product receives FDA approval as expected.
Biopure’s stock has bounced around a lot over the past year along with other stocks in the sector, many of which were hammered particularly hard in February and March. Analysts say there could be more volatility in the stock over the short run, which should abate once the outcome of the current testing and marketing approval process becomes more certain.
“This isn’t a stock for widows and orphans,” says Larry Neibor, an analyst with Robert W. Baird & Co. in Milwaukee. “But all of the other contenders in this area have failed to make it through clinical tests due to safety concerns, and that’s obviously not a problem here.”
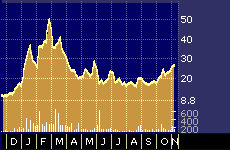
One-year performance of Biopure, Inc.
Neibor notes that several previous high-profile attempts to test new blood substitutes in human patients were halted midway through the process, due to concerns over jeopardizing the safety of the subjects. Biopure, by contrast, has successfully jumped that hurdle by moving through the human testing phase thus far without incident. In addition, the firm is already using a related Hemopure product, called Oxyglobin, in veterinary medicine applications where its safety and efficacy have already been well established.
One of the breakthrough advantages of Hemopure’s product is that it is both species and blood-type agnostic. The company says its product can be used in both animals and humans without regard to specific blood type.
The product’s features make it an ideal candidate for a variety of medical applications, such as use on military battlefields, in elective surgery or by emergency medical personnel such as ambulance crews who aren’t currently able to stock fresh blood supplies. There is an estimated shortage of about 250,000 thousand units of blood per year in the Unites States, representing about 2% of the overall supply, according to estimates by J.P Morgan.
Estimates of the potential overall dollar value of the market are hard to come by since it doesn’t exist yet. Biopure is, however, charging about $125 for every unit of Oxyglobin purchased by vets. The company says it is able to manufacture 100,000 units of Hemopure a year and has plans to boost that to 500,000 with the next three to four years.
Neibor has a $60 12-month price target on Biopure’s stock, which he rates “buy-speculative,” his firm’s most-enthusiastic recommendation for investors who can tolerate some degree of risk.
At least three other companies are also conducting tests on human blood substitutes. Biopure, however, is the only company developing a human blood substitute based on a manipulation of low-cost, always-available cow blood. The other firms, which include Alliance Pharmaceuticals Corp. {ALLP}, Canada-based Hemosol Inc. {TSE: HML} and Northfield Laboratories Inc. {NFLD} are using a variety of other approaches, which may also prove valid, that are formulated from either harder-to-obtain expired human blood or recombinant technologies that have often proven problematic in the past.
The problem with expired human blood is there is so little of it available, given current blood-supply shortages, which usually means most blood is used well before it has a chance to expire. Likewise, recombinant technologies can yield less-expensive synthetic alternatives but have, unfortunately, also proven prone to causing unwanted side effects of the type that can be less common with biologically based substances.
“Alliance Pharmaceuticals is in the hunt with a product that could be cheaper to produce,” says Alfred Mansour, Ph.D., chairman and CEO of BiotechWatch.com, an online service based in Franklin, Mich., which tracks the progress of biotech firms.
But Mansour notes that Alliance’s product is technically not a blood substitute at all but rather is designed to prevent the need for blood transfusions in the first place. Hemosol’s product might also be competitive, he says, but a recent trial in Canada left investors confused when the company’s original results, which were later amended, didn’t show a reduction in the need for blood transfusions.
There have also been troubles at Northfield, with major shareholders of that firm publicly questioning management’s ability to successfully bring its new product to market, according to Mansour.
“Biopure’s results thus far have been positive,” Mansour says. “Therefore, the likelihood of clinical success is quite good.”
Development of a substitute for human blood is something of a holy grail within the biotech industry. There have been several conspicuous failures in recent years, most notably at the once highflying Somatogen, which was acquired by Baxter International Inc. {BAX} in 1998 before it became fully apparent the company’s approach wasn’t working. But many biotech companies have continued to work on the problem, driven on by increasing shortages of human blood supplies, which have just a 42-day shelf life when properly refrigerated.
Biopure’s product, by contrast, has at least a two-year shelf life and doesn’t require any refrigeration.
“Biopure has been operating mostly under the radar screen, particularly with institutional accounts,” says Phil Nalbone, biotech analyst at Salomon Smith Barney in San Francisco. “But we’re very enthusiastically recommending the stock ahead of the key milestones that typically drive valuation in medical stocks.”
The analysts say that if Biopure wins FDA approval as expected sometime next year, its stock performance might approach that of other biotech stocks, such as IDEC Pharmaceuticals Corp. {IDPH}, Immunex Corp. {IMNX} and Medimmune Inc. {MEDI}, all of which soared fourfold or more after their new products won FDA approval over the past few years.
Jim McCamant, publisher of The Medical Stock Technology Newsletter, based in Berkeley, Calif., says he is cautiously optimistic about Biopure’s prospects.
But the big disappointment at Somatogen left McCamant somewhat wary.
“If they announce the results people are expecting the stock will go up,” McCamant says. “But typically you don’t really know until they have done all the analysis, which is what’s happening now. They’ll also have to prove the can make it in large quantities. But it could be a very good speculative play.”
Franklin Berger, an analyst at J. P. Morgan in New York, upgraded Biopure’s stock to “buy” from “market perform” last week, raising his 12-month price target from 30 to 40 after the company announced the hiring of Dr. Michael Hensley as head of clinical and regulatory affairs at the company. Berger says Hensley has the “skill sets and experience required” to guide the company through the final steps involved in winning FDA approval.
“One of the biggest users of blood is for hip and knee replacements,” Berger adds. “And guess what’s happening as all the baby boomers get older? You get older hips and older knees that need to be replaced. What Biopure offers is a net new addition to the blood supply. The only question now is how safe it is, and we expect to get the thumbs up on that in January or February.”
Full commercial approval would then be expected by year-end 2001, assuming the company requests and receives expedited processing from the FDA, as anticipated.


