
A Potential Biotech Blockbuster
A Potential Biotech Blockbuster
By Hal Plotkin
CNBC.com Silicon Valley Correspondent
Apr 13, 2001 08:00 AM
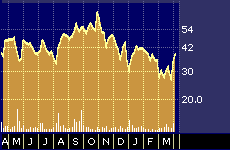
ImClone Systems 52-week stock performance
Positive preliminary clinical data released this week by ImClone Systems Inc. {IMCL} touched off a buying spree that pushed the volatile stock up roughly 10 percent over two days and led to predictions it will move up even further in anticipation of a key scientific research meeting in May where more complete and definitive clinical results are set to be announced.
“In our view ImClone is poised to become one of the next commercial success stories in biotechnology,” wrote Douglas Lind, M.D., biotechnology analyst at Morgan Stanley Dean Witter, based in New York, in a research note published on Wednesday, just after the latest clinical news was released.
Lind has an “outperform” rating on the stock along with a $57 one-year and $104 three-year price target, which represents 80 times his estimate of the firm’s earnings per share at the end of that period.
Although that multiple may seem high, industry experts point out that it is in keeping with the pop enjoyed by other biotech firms that have introduced important new therapeutics in recent years, such as IDEC Pharmaceuticals Corporation {IDPH}, which introduced a somewhat similar compound called Rituxan to treat lymphoma in 1997, and which is now trading at a P/E in excess of 146.
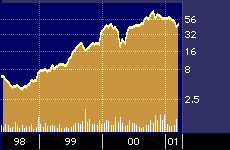
IDEC Pharmaceuticals 3-year stock performance
“The market potential for ImClone’s new drug is five times larger than Rituxan, and you saw what Rituxan did for IDEC [Pharmaceuticals],” says Berkeley, California-based Jim McCamant, who runs the American Health Care hedge fund and who also publishes the Medical Technology Stock Newsletter.
Earlier this week, The American Society of Clinical Oncologists published abstracts of the research papers slated to be delivered at its May 12 annual meeting in San Francisco. Although more complete and up-to-date data is expected at the meeting, ImClone’s abstract of its presentation indicated a 17 percent response rate among refractory colorectal cancer patients given its C225 compound in combination with other treatments.
The term refractory means those cancers were not responding to pre-existing treatments. Any response rate greater than 15 percent is likely to lead to expedited approval from the Food and Drug Administration to begin marketing the new drug given the severity of the often-fatal disease, according to several biotech experts.
ImClone’s stock has come under attack by short-sellers in recent months, which is a common development in the biotech industry, where hype often gets ahead of actual corporate performance given the long lag-time associated with new drug development.
By mid-March, for example, the last date for which reliable numbers are available, investors had shorted 5.7 million shares of the stock, according to McCamant. Investors who sell a stock short are betting it will go down.
“I think the shorts are making a big mistake on this one,” says McCamant. “They don’t seem to understand how the FDA works these days.”
McCamant says he has seen at least one short selling recommendation that predicted it would be years before ImClone won approval for its new compound. That prediction, he says, was based on the idea that the FDA would wait to see the long-term survival rates of patients treated with the compound before issuing an approval to start selling the drug.
But McCamant and others say ImClone is likely to be able to take advantage of expedited FDA approval procedures set up during the Clinton administration that bring new drugs that treat fatal diseases such as AIDS and cancer to market faster if they are showing certain signs of clinical success. In the case of cancer treatments, those signs, which are called “surrogate markers,” include a reduction of 50 percent or more in tumor size.
“It looks like the American Society of Clinical Oncologists (ASCO) May meeting will be the beginning of the marketing,” says McCamant, who expects to see the company win expedited approval to start selling the drug by the end of this year or early next year. Similar trails of the compound are also underway in connection with head, neck, lung, and pancreatic cancers.
“ASCO is an extremely important meeting,” says Karen Bernstein, editor of BioCentury, a Belmont, California-based biotechnology newsletter. Bernstein prefers not to make public comments on specific stocks or companies, but she confirms that industry watchers are keeping a very close eye on clinical news about ImClone’s progress.
“In our view, ImClone is one of the highest probability product development bets in biotechnology,” adds Dr. Lind, of Morgan Stanley Dean Witter.
That doesn’t mean the new compound or the stock is a sure bet. The experts acknowledge that it’s always possible the final data will not be as positive as it now appears, or that other companies will come up with competing drugs and approaches that prove more effective.
There are at least two other biotech firms, Genentech Inc. {DNA} and AstraZeneca PL {AZN}, which are developing similar drugs for colorectal cancer, although recent data indicates those drugs are 12 to 18 months behind ImClone in terms of human testing and also appear to have more significant side effects, most notably diarrhea.
McCamant, a veteran industry watcher with more than two decades of experience, is betting the news at ASCO will be even better than the preliminary abstract given the additional time the company will have to report more results from patients now being treated.
Dr. Lind also says he expects to see the company submit its final data on C225 for FDA Fast Track review during the current quarter, with approval following in roughly six months.
“Risks generally applicable to the biotechnology sector should be considered,” he says. But he adds that like McCamant he is hopeful the new compound will find many other applications.
“Because the product targets the EGF receptor, which is uniquely found on one third of all solid tissue cancers, the potential market may expand well beyond head, neck, and colorectal cancers,” he says.


