
Sequenom IPO Shoots Higher in Debut
Sequenom IPO Shoots Higher in Debut
by Hal Plotkin
Silicon Valley Correspondent
Investors have welcomed Sequenom Inc.’s {SQNM} initial public offering enthusiastically, sending shares up past 78.
“The company is in the hottest part of the genomics sector,” says Jim McCamant, editor of Medical Technology Stock Letter, based in Berkeley, Calif.
Late Monday, 5 million shares of the company were priced at 26 a share, above the most-recent offering range of 23 to 25. That is sharply higher than the original range of 16 to 18.
“Coming off a marvelous first three weeks in January, the biotech sector is still warm,” McCamant says. “Others in the same sector have all gone on to big premiums. As long as we have a lot of speculative money in the market moving into the biotech sector, it’s going to be reasonably easy to do these deals.”
Much of that fresh money, McCamant says, comes from Internet investors who are now shopping around for new places to put their winnings to work.
McCamant says the performance of rival Affymetrix Inc.’s {AFFX} stock provides an indication of the potential for a reasonably high valuation of Sequenom’s stock.
“Affymetrix is the leader,” McCamant says. “With something like a $5 billion market cap, anything else looks cheap in comparison.”
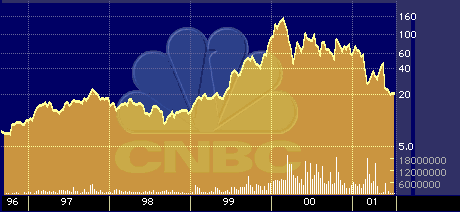
Affymetrix 52-Week Stock-Performance Chart
San Diego-based Sequenom is among a growing number of companies that have developed proprietary technology to conduct single nucleotide polymorphism (SNP) genotyping. The technology helps scientists uncover data about subtle genetic variations that are expected to lead to new treatments for diseases as well as advances in agricultural sciences and livestock management.
Somewhere between 10 million to 1 billion SNP measurements are typically required to find the association of genes to a particular disease, according to an estimate by John Stuelpnagel, CEO of Illumina Inc., a competing privately held SNP-analysis firm based in San Diego. Stuelpnagel’s estimate appeared in a recent issue of Biocentury, a leading biotechnology industry newsletter.
“SNP is going to become a commodity business,” says Karen Bernstein, the editor of Biocentury. “Right now, it’s hard to tell which of the many approaches to the SNP market will be most successful.”
Several other companies, such as Hyseq Inc. {HYSQ}, Incyte Pharmaceuticals Inc. {INCY}, Kiva Genetics Inc., and Orchid Biocomputer Inc., in addition to Sequenom, Affymetrix, and Illumina, are all developing, or in some cases have already begun selling, a variety of different SNP-analysis tools.
“What I find appealing about Sequenom is their use of mass spectrometry,” Bernstein says. “It’s really powerful and qualitative, and they seem to have a lead in that area. I happen to think it’s a pretty cool company.”
According to statements by company officials, Sequenom’s technology provides accurate data at lower costs than more-common methods currently in use, such as fluorescence, while simultaneously returning more information about each individual experiment.
Bernstein says Sequenom’s leadership using such techniques could give the company an advantage moving forward in what is expected to become a very competitive, price-sensitive industry. “That’s the theory,” she says.
Sequenom began beta-testing its products in association with leading academic institutions, including Boston University, Johns Hopkins University and the University of Hamburg, in July 1999. A formal commercial launch of the firm’s products is anticipated almost simultaneously with the IPO.
Over time, Sequenom plans to develop new knowledge-based products that combine the information developed by users of the firm’s technology. Sequenom’s S-1, the firm’s official pre-IPO filing with the Securities and Exchange Commission, acknowledges those plans could fail to materialize if third-party customers decide they would prefer not to collaborate with the firm.
Another risk factor for Sequenom, along with nearly all the other players in the same market, involves somewhat unsettled intellectual property law as it pertains to the products of genetic investigations. Sequenom’s S-1, for example, notes that an unnamed firm has already made claims of patent infringement which Sequenom officials maintain aren’t valid.
“That’s not at all unusual,” McCamant says. “The intellectual property in this area is clearly a bit fuzzy.”
Although Sequenom’s IPO is expected to be favorably received, analysts say investors should keep a careful eye on the customer lists of the different SNP competitors moving forward to see which of the firms stands the best chance of success over the long run.
“It’s too early to tell right now,” Bernstein says. “But the proof is going to come by looking at the customers of these firms and seeing which customers use which solutions.”
Sequenom posted a loss of $15.3 million after taking in $81,000 in research grants during the nine months ended Sept. 30, as compared with a loss of $6 million during the same period a year earlier, when the company received $126,000 in research-grant income.
Sequenom has received more than $25 million in start-up funds from a consortium of life-sciences venture-capital funds. The firm has an ongoing collaboration with Brucker-Franazen Analytik, a German equipment manufacturer, which is providing the necessary mass spectrometry equipment.


