
Sorting Through a Slew of Biotech IPOs
Sorting Through a Slew of Biotech IPOs
By Hal Plotkin
Silicon Valley Correspondent
The Medicines Co. {MDCO} and Lion Bioscience Inc. are expected to be the cream of this week’s crop of eight biotechnology initial public offerings, but analysts warn that investments in early-stage biotech firms are usually not for the faint of heart.
“I think we’re going to see the volatility continue,” says Matt Berry, an analyst with the Medical Technology Stock Letter, based in Berkeley, Calif.
In fact, Berry says steering clear of most biotech stocks in the immediate IPO after-market has been a key to the success of his newsletter’s recommended model and aggressive biotech portfolios, which have surged by 1,529 percent and 5,654 percent, respectively, since they were started in 1987.
“We tend to wait at least until well into the second year before we recommend a stock,” Berry says. “The experience has been that they go below their offering price sometime within two years.”
Berry says that in most cases it is a long time between when a biotech company goes public and when, if ever, it begins posting the steady earnings gains that make for a dependable, reliable, upwardly mobile stock. In the interim, biotech stocks, particularly many new issues, are subject to wild price swings as investor enthusiasm waxes and wanes with each fresh tidbit of clinical news or research results.
|
Best & Worst Biotech IPOs since 1998 |
||||||||
| Issuer | Symbol | Issue Date |
Proceeds Amt – in this Mkt ($ Mil) |
Offer Price |
Offer Price Adj. for Splits |
Stock price at Close of Offer/1st Trade |
Y’day’s Stock Price |
%Chng Y’day’s Price to Offer Price |
|
Best |
||||||||
| United Therapeutics Corp |
UTHR | 06/17/99 | 54.0 | 12.000 | 12.000 | Zero | 112.875 | 840.625 |
| CuraGen Corp | CRGN | 03/18/98 | 34.5 | 11.500 | 5.750 | 12.50 | 39.50 | 586.957 |
| King Pharmaceuticals Inc |
KING | 06/25/98 | 87.5 | 14.000 | 6.222 | 14.00 | 36.813 | 491.638 |
|
Worst |
||||||||
| Women First Healthcare Inc |
WFHC | 06/28/99 | 49.5 | 11.000 | 11.000 | 11.56 | 1.00 | -90.909 |
| Horizon Medical Products Inc | HMPS | 04/14/98 | 50.4 | 14.500 | 14.500 | 15.56 | 2.00 | -86.207 |
| Vantagemed Corp |
VMDC | 02/15/00 | 36.0 | 12.000 | 12.000 | 10.06 | 2.125 | -82.292 |
| Source: Thomson Financial Securities Data Data as of 8/8/2000 |
||||||||
Berry says this week’s expected flood of new issues will make it tougher swimming for all the new entrants crowding into the biotech pool. “It’s going to make it harder to sustain upside with all that stock out there,” he says. “But there are good companies going public.”
“The strongest companies are the ones with drugs in late-stage development, or the companies that are selling tools that have the partnerships they need in place,” says Alfred Mansour, Ph.D., chairman and chief executive officer of BiotechWatch.com, an online service based in Franklin, Mich., that tracks the progress of biotech firms.
Nadine Wong, who edits the Biotech Sage Report, an industry newsletter based in Beaverton, Ore., agrees that the volatility will continue but says the sector is making steady progress on its march to overall profitability.
“There’s a lot of hype,” Wong agrees. “But the hype is going to pan out. I think we’re going to see a lot of excitement for the biotech stocks, as a group, in September, when Celera Genomics Group {CRA} and the Human Genome Project publish their final papers [mapping the human genome]. That’s going to get people excited all over again.”
Wong says volatility in the biotech sector attracts momentum investors, who rapidly get in and out of the stocks, which further increases their volatility. “They’re not loyal investors, just out to make a fast buck,” she says. “You can lose a lot of money because of that if you’re not careful.”
Instead, Wong says, investors who want to get in on biotech’s long-term upside potential should keep an eye on the stocks of companies that have the most-solid, near-term commercial prospects.
“You have to be prepared to be very, very patient,” Wong says. “In six months, you’ll probably see many of the new IPOs come down again.”
Overall, analysts warn that dabbling in freshly offered biotech issues could be a very risky business.
“The longstanding rule is to buy on anticipation and sell on realization,” says David Menlow, an analyst at the IPO Financial Network, based in Millburn, N.J.
“The big problem, though, is that most investors are uncomfortable waiting as long as they have to wait for a biotech company. That periodically brings the stocks down on a very significant basis.”
Mansour says Medicines and Lion Biosciences are this week’s strongest new biotech contenders.
Medicines
Based in Cambridge, Mass., Medicines is expected to receive final approval shortly from the Food and Drug Administration to begin selling its first product, Angiomax, which is used to treat heart disease. The company has several other heart-disease related compounds in development, including one other in the final stage of testing, Phase III, and one that is nearing the end of Phase II trials.
“The Medicines Company is the most-mature product development play this week,” Mansour says.
The company’s success is noteworthy, he says, because it obtained the right to its first product from another firm, Biogen Inc. {BGEN}, whose early test results on the same compound proved disappointing.
“They took a drug that another company had essentially given up on and refined the testing,” Mansour adds.
After more research, the drug’s desired effect in a smaller, but still significant, subset of patients became more apparent.
It is an important development in a huge market, Mansour says, since heart disease still ranks as the nation’s No 1. killer.
“The results you get in clinical trials are entirely dependent on how the trails are conducted,” Mansour adds. “They’ll probably share markets with others for a while, and will need to differentiate their products. But this shows they can get products to market.”
Late Monday, Medicines priced its 5 million share offering at $16 a share, the top of its $14 to $16 price range. The stock debuted on Tuesday at 20 7/8 and has traded as high as 22 1/4.
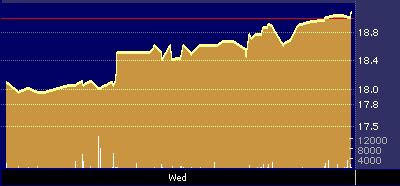
Medicines post-IPO stock performance
Lion Bioscience
Based in Heidelberg, Germany, Lion Bioscience develops and sells data-analysis and information-management systems for the life-sciences and health-care industries.
“I think Lion will be strong because of the interest in genomics and the number of strong partnering deals already in place,” Mansour says.
The company has corporate partnerships, including potentially lucrative royalty arrangements, with firms such as Bayer AG {BAYZY}, Boehringer Ingelheim, Paradigm Genetics Inc. {PDGM} and Pharmacia Corp. {PHA}.
Mansour says Lion Bioscience’s technology focuses on an absolutely critical part of the research and product development food chain: identifying possible new drug candidates.
Mansour says the firm’s first product, called the arraySCOUT 1.1, has applications across the entire newly evolving spectrum of genetically based medicine.
“There are many, many potential applications,” Mansour adds. “Typically, the company will work with another firm, or firms, to target individual product-development areas. They’ll focus on a specific area; it just depends what their partners are working on. The technology could be used very broadly in the drug-development process.”
Here’s a rundown of the other biotech stocks slated to begin trading over the coming days:
AtheroGenics Inc.
Based in Alpharetta, Ga., AtheroGenics has two drugs in development, both in Phase II trials, aimed at treating chronic inflammatory diseases such as asthma, arthritis and asatherosclerosis, which is a form of coronary artery disease.
The company has an exclusive worldwide license agreement with Schering-Plough Corp. {SGP} to develop and commercialize its first product, AGI-1067. The agreement calls for AtheroGenics to receive as much as $189 million from Schering-Plough in licensing and milestone payments, excluding royalties and development costs, should the firm’s current drug-development program prove successful.
Esperion Therapeutics Inc.
Based in Ann Arbor, Mich., Esperion plans to start Phase I testing of a drug aimed at cardiovascular disease by the end of this year.
“Esperion is an interesting situation,” Mansour says. “They’re dealing with the problem in a unique way, but it’s still very early. It will probably be at least five to seven years before they’ll able to enter the market. That means they’re going to need to raise money for the next five or maybe ten years.”
Esperion’s target is the plaque lining blood vessels that causes heart disease, and, often-fatal heart attacks. Unlike compounds that eliminate cholesterol from the bloodsteam, Esperion’s scientists hope to be able to use their new drug to actually break up and dissolve plaque that has already adhered to blood vessel walls.
“There’s a big market for that if they can get there,” Mansour says.
Ista Pharmaceuticals Inc.
Based in Irvine, Calif., Ista Pharmaceuticals has a drug in Phase III trials aimed at an eye disease called vitreous hemorrhage. The firm also has a compound in Phase II trials targeted at diabetic retinopathy, which can threaten the vision of patients with diabetes.
Analysts say they are impressed with the company’s progress but add that the overall size of the market addressed by the firm may not be large enough to make it stand out in the increasingly crowded biotech sector.
Large Scale Biology Corp.
Based in Vacaville, Calif., Large Scale Biology has a patent portfolio that it says protects its proprietary technologies, called ProGEx and GENEWARE, which are aimed at a broad range of commercial opportunities.
The products are used to identify candidates for drug development.
The company has an exclusive contract with The Dow Chemical Co. {DOW} and its subsidiary Dow AgroSciences LLC, aimed at identifying commercially significant genes for agricultural and industrial uses that will be marketed by Dow Chemical and its affiliates. Dow Chemical can elect to continue the collaboration beyond September 2001, when the current agreement expires. Large Scale Biology derived $14.2 million in revenues from Dow Chemical in 1999.
The firm’s official pre-IPO filing with the Securities and Exchange Commission, called an S-1, claims other ongoing “collaborations with pharmaceutical, biotechnology, chemical and other life-sciences companies, as well as research institutions and government,” but lists no other specific collaborations other than its arrangement with Dow Chemical.
Likewise, the “Alliances and Clients” section of the company’s Web site leads to a page that is “currently under construction.”
Regeneration Technologies Inc.
Based in Alachua, Fla., Regeneration Technologies markets products from human cadavers that are used to improve surgical outcomes.
The company has several products already on the market, which generated sales of $73 million in 1999, along with a profit of $2.9 million over the same period. Regeneration Technologies plans to use the proceeds from its IPO to construct new facilities and further expand operations.
“It’s hard to get too excited about a company marketing products from cadavers,” Mansour says. “You can already get a good sense of what that business is like from how they’re doing, and it doesn’t look like we’ll see any big surprises there.”
Telik Inc.
Based in South San Francisco, Calif., Telik, is in the early stage, or Phase I, of testing clinical compounds aimed at cancers that have resisted standard treatment. The company’s first drug candidate, a “small molecule” compound currently called TLK286, is now undergoing its first trials in human subjects while another, TLK199, is slated to begin similar testing before the end of the year.
“Companies in the earlier stages of testing could be promising,” Mansour says. “But you’ve got to think of it as an attrition problem. Maybe 50 percent of products in Phase I go on to the next stage of testing, then, maybe at best, less than 70 percent of the Phase II trials go on. The further you go the better your chances,” he says.
Investors who back biotech companies during the early stages of clinical tests could, Mansour warns, wind up with a handful of nothing.
“The likelihood of that happening is higher with companies in pre-clinical or early stages,” he says.


