
CV Therapeutics: A Heart-Stopping Stock
CV Therapeutics: A Heart-Stopping Stock
by Hal Plotkin
Silicon Valley Correspondent
Although big drug stocks led the drug/biotechnology sector’s rebound this week, several analysts say they’re more excited about the stock of a lesser-known biomedical firm, CV Therapeutics Inc. {CVTX}.
“We definitely see it getting back into the $20s,” says Franklin M. Berger, an analyst at J.P. Morgan Securities Inc., based in New York, one of the firm’s underwriters. “CV has a novel pharmaceutical franchise and is about to begin a long period of profitability.”
Palo Alto, Calif.-based CV Therapeutics is a biopharmaceutical company focused on developing new treatments for cardiovascular disease. The company’s stock nearly quadrupled last August after CV Therapeutics announced favorable initial results from Phase III clinical trails of its new drug, ranolazine, which treats angina, a form of chest pain associated with heart disease.
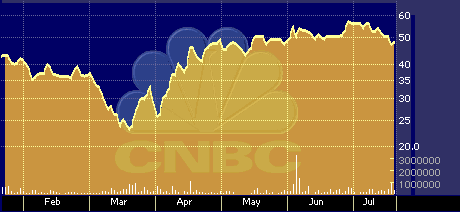
CVTX six-month stock performance chart
Earlier this month, BancBoston Robertson Stephens senior biotechnology analyst Jay B. Silverman initiated coverage of the stock with a “buy” rating.
“In our view, angina represents an enormous market opportunity, with more than 7 million Americans affected,” Silverman said in releasing his recommendation. “We believe there is substantial potential for ranolazine.” BancBoston Robertson Stephens also acted as an underwriter for CV’s most recent stock sale.
Silverman set a year-end 2000 price target of $25 for the stock. It’s based, he says, on 40 times his projected 2003 earnings-per-share estimate of $0.97, discounted 25 percent. Silverman figures ranolazine sales should hit $36 million in 2002, following a mid-2002 launch, and climb to $110 million in 2003. “Longer term, we believe CV Therapeutics could be one of the best performing biotech stocks,” he says.
CV Therapeutics went public in November 1996 selling 1.75 million shares at $8. In January 1998, the firm sold an additional 2.3 million shares at $8.25 each. The company sold 5 million more shares at $12 each earlier this month on Oct. 6.
Despite its increasing supply, the stock has fared quite respectably over the past 12-months, notwithstanding it’s post-September slide. Measured on an annual basis, CV Therapeutics ranks 30th in its sector, posting a 117 percent gain over the past year.
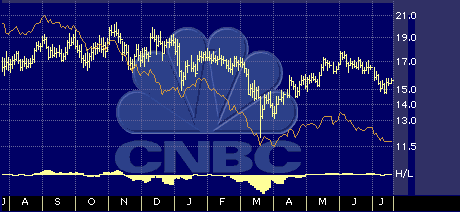
One-year drugs/biotechnology sector performance chart
Orange line: Daily Advance Decline line
Yellow line: 6 Day Daily High-Low line.
Several factors are contributing to analyst optimism about CV Therapeutics’s stock.
The most important is the positive report released by the company concerning the completion of the first half of its phase III ranolazine trials. Those tests determined the drug, when used alone, significantly increased cardiovascular performance. The company is currently conducting additional tests to determine how the drug interacts with other medications, which is expected to be the final part of the Phase III clinical trials. (Phase I and Phase II trials don’t involve human subjects).
Tricia Nagle, managing editor of the Drug and Market Development newsletter, based in Southborough, Mass., says the fact that ranolazine has reached the second part of Phase III trials is “very encouraging.” Nagle says that more than 90 percent of all new biopharmaceuticals under development never reach even the first part of Phase III trials. “They’re pretty far along,” she says. “In most cases the drug interaction study is not that big a deal at that point. I can’t say for sure, but it probably won’t be a problem.”
But Jim McCamant, editor of the Medical Technology Stock Letter, based in Berkeley, Calif., says investors shouldn’t entirely discount the possibility that prolems will emerge during ranolazine’s final clinical tests.
“The farther along you are, the lower the risks, but we have had some spectacular failures in Phase III trials,” McCamant says. Notwithstanding that warning, however, McCamant says the company’s results so far “sound promising and justify a change in the stock price.”
CV Therapeutics is in an excellent position if ranolozine does pass its final round of tests. The drug has a unique mechanism of action that could make it a preferred therapeutic. Current angina therapies, which include beta blockers, calcium channel blockers, and nitrates, all treat angina by lowering the heart rate, blood pressure, or the pumping of the heart muscle. Ranolazine, by contrast, works by helping the heart muscle work more efficiently.
“Because of [ranolazine’s] unique method of action, patients may be able to find relief from the painful attacks of angina without some of the unwanted effects of current anti-anginal drugs,” said Louis G. Lange, M.D., Ph.D., and chairman and CEO of CV Therapeutics, in announcing the initial phase III results.
CV Therapeutics is also moving to make the most of its potentially lucrative new compound. In May, the company signed an agreement with Innovex Inc., a subsidiary of Durham, N.C.-based Quintiles Transnational Corp. {QTRN}, to commercial ranolazine in the United States. The agreement, essentially an outsourcing arrangement, allows CV to retain more profits than it would have retained had the company made a more traditional distribution agreement with a major pharmaceutical firm.
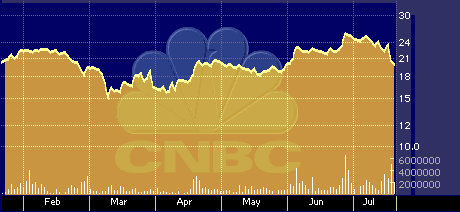
QTRN six-month stock performance chart
“It’s pretty new that a biotechnology company is doing that kind of deal with a contract research organization,” Nagle says. “But it’s a trend. Biotech firms get a much better deal that way.”
It’s always possible ranolazine could still disappoint investors by stumbling during its final trials, or be eclipsed by even better drugs developed by other firms. However, since more than 65 percent of all angina patients currently use two or more drugs, it’s likely the compound will encounter strong market demand if it does become available in mid-2002 as expected.
“It’s a humongous market,” Nagle says. “There’s a lot of potential for the drug.”
CV Therapeutics reported a loss of $5.3 million for the second quarter of 1999 as compared with a loss of $3.8 million for the same quarter in 1998.


